UK FMD 2021 Changes
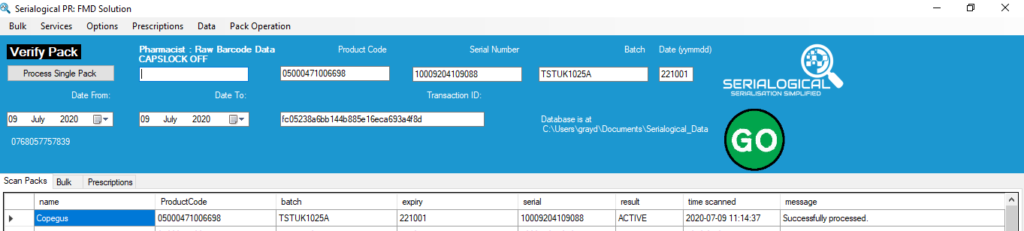
With the end of the transition period on 31st December 2020 the UK will leave the EU. Mainland UK then becomes a 3rd country, not part of the EU FMD system. End users such as pharmacists, hospitals and wholesalers and will no longer be able to verify or decommission FMD labelled medicines. Northern Ireland will …