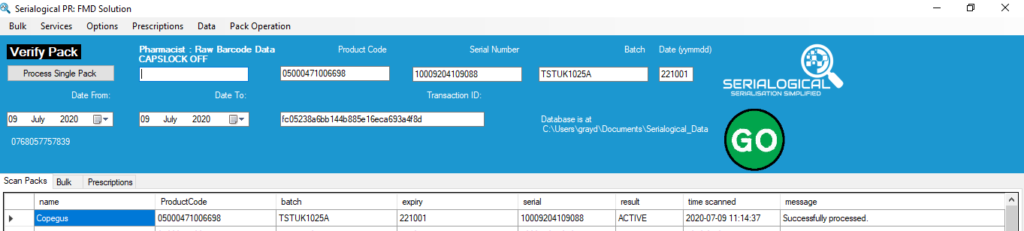
All EU FMD pack codes include 4 compulsory elements, the Product Code, Serial Number, Batch and Expiry Date. The combination of these 4 elements uniquely identify a pack.
SecurMed are ramping up for a 5th Element to be included within UK FMD codes. This is the Actual Medicinal Product Pack (AMPP) code. It fits into the existing optional NHRN (National Health Registration Number) slot which is used by some countries to reimburse dispensers for supply of a product. The new NHRN code (AMPP) in the UK is specified as:
- Prefix 7XX (the X being any number)
- Numbers only
- Length 6-18 digits
- Typical length 16 or 17 digits
The FMD code components can be in any order. The NHRN element could be placed between existing elements. In such a case it would be followed by the Global Separator (<GS>) to allow the scanner reading the code to know where the NHRN stops and the next element ends. It is probable that for existing packs the NHRN would be likely to be the final element in a FMD as that would involve the least disruption of the packing line.
SecurMed require manufacturers to upload the new NHRN code with their product master data. This is information linked to the product code and also includes details such as the manufacturer name, product name and brief product details. At present the NHRN code does not need to be present within the UK FMD codes although it might be present as UK manufacturers prepare for its mandatory inclusion. Our Serialogical software will detect the NHRN code and output it for information. Inclusion of an NHRN code will not affect verification or dispensing of otherwise valid codes.
At present AMPP codes are used within the NHS Dictionary of Medicines and Devices (dm+d). The Dictionary includes details of pack contents and suppliers for prescription medicines. The DMDBrowser is an on-line portal to dm+d details which can be browsed without needing log on credentials.
Identifiers from the dm+d are used within the UK electronic prescription system. In an electronic prescription a product is prescribed, in many cases this would be available from more than one manufacturer. Within the FMD a unique pack is identified, the dm+d information is not specific enough for the FMD. The manufacturer and packing details can already be tracked down from the existing 4-part FMD code.
Some added value must have been envisaged in adding the NHRN data within the UK. The electronic prescription system does not include enough data to dispense a specific pack. On the other hand because the FMD data includes a dm+d identifier a FMD scan could be linked to check that the packet scanned matches that on an electronic prescription. This would be an electronic safeguard to the physical check that the pharmacist makes when dispensing a product and ensuring that it matches the existing prescription.